


Become an Allurion Partner
Support your patients with proven, non-invasive, weight loss programme that combines medical innovation, digital tools and end-to-end clinic support.
- 180,000 balloons placed
- Offered in 80+ countries
- Placed during a 20-minute outpatient visit ¹
- 10 to 15% total body weight loss ⁴*
Allurion Virtual Care Suite
Smart patient monitoring
A key component of the Allurion Program, the Allurion Virtual Care Suite is a dynamic weight-loss management suite powered by artificial intelligence and designed to maximize your patient outcomes and satisfaction.
Remote patient monitoring, telehealth and care team collaboration - all under the one digital umbrella.


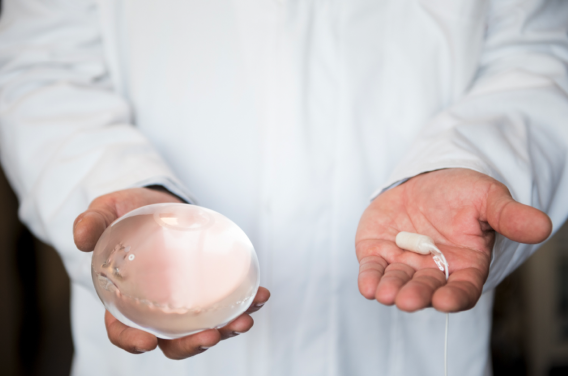
The future of weight loss, delivered through your clinic
The Allurion Program combines the world's first and only swallowable gastric balloon that requires no surgery, endoscopy or anaesthesia**, with a comprehensive behaviour change and digital care platform. It empowers healthcare providers to deliver clinically proven weight loss of up to 10 - 15% in just four months - while supporting long-term behaviour change, muscle preservation and patient satisfaction.
The Allurion Program is addressing the unmet needs of your patients.

more patients would consider the Allurion Balloon compared to the number who would consider bariatric surgery.⁵
Hear from other professionals
Trusted by world-class clinics


